Researchers find new lead for disarming antibiotic-resistant bacteria
Bacteria-infecting viruses may have novel uses in medicine
A virus can stop bacteria from sharing genes for antibiotic resistance among themselves, Texas A&M AgriLife researchers have discovered. The results hint at new ways to treat infections and describe a new feature of a highly diverse, largely unexplored part of the biosphere.

The study, published recently in Proceedings of the National Academy of Sciences, was led by Lanying Zeng, Ph.D., associate professor in the Texas A&M College of Agriculture and Life Sciences Department of Biochemistry and Biophysics.
How some phages infect bacteria
Viruses that only infect bacteria are called bacteriophages, or phages for short. Phages are the most numerous biological entities on Earth. Soil is rife with phages, as is the human gut, and phages that infect and destroy bacteria have found promising uses in combating antibiotic-resistant bacterial infections.
Some phages only infect bacteria whose surface contains cylindrical structures called pili. Named after the Latin word pilus, for spear, pili allow bacteria to transfer genes for advantageous traits, such as drug resistance, and enhance bacteria’s ability to move and to attack host cells. Because of pili’s link to bacterial virulence, researchers have wondered whether new medications could be created to inactivate this feature. While the ways bacteria benefit from pili are clear, how phages use pili to infect bacteria has remained elusive.
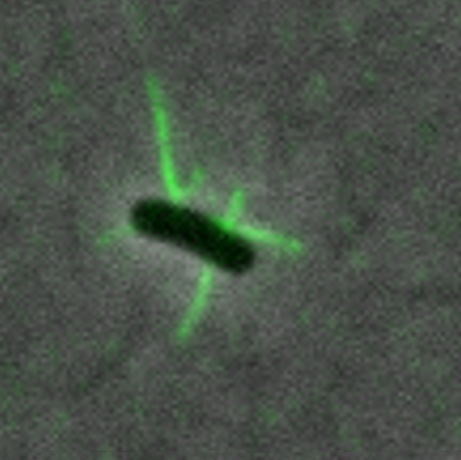
Phage competition
Zeng’s team used fluorescence microscopy to delve into how a phage, MS2, enters an E. coli cell. The researchers created MS2 phages that fluoresce and are fully infectious and stable. The phages attach to pili on E. coli cells, making the pili visible through a fluorescence microscope.
Through a series of experiments, Zeng, her graduate student Laith Harb, and the other coauthors obtained a detailed description of what happens when an MS2 phage infects an E. coli.
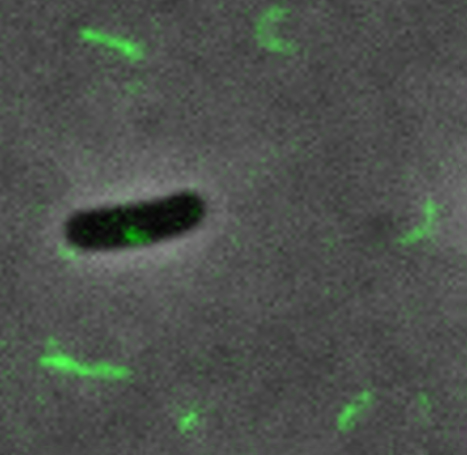
The team discovered that after a phage attaches to a pilus, the pilus retracts, bringing the phage to the bacterial cell surface. The pilus then breaks off behind the phage. Whereas healthy E. coli replenish broken pili, cells infected by MS2 do not. In this way, other phages are prevented from infecting the same cell. The first phage to reach the cell gains a competitive advantage.
“It’s like, ‘OK, this cell is mine.’ Phages set up their own territory,” said Zeng, who is a core faculty member of the Center for Phage Technology, a part of Texas A&M AgriLife Research.
Because the phenomenon gives such a boost to the infecting phage, this occurrence may be widespread among other phage strains that employ pili to infect bacteria, Zeng added.
New ideas for medicine
The results may be of use in medicine, Zeng said. First, using phages to decrease bacterial virulence may give the immune system time to fight off an infection. Second, the results point to a way of dealing with infections that may be gentler for patients than antibiotics or than using phage therapy to destroy bacteria.
“One advantage of our method versus traditional phage therapy is that you do not kill the cell, you just disarm it,” Zeng said. “Killing the cell may cause a problem, because inside the cell you may have a toxin that could be released into the host.”
Phages that target pili could also reinforce the action of antibiotics. Some bacterial infections only respond to high doses of antibiotics, which can cause side effects. Adding phages to the mix may allow doctors to decrease the needed antibiotic dosage.



