Celebrating Ryland Young’s career, advances in phage applications
New biochemistry lecture series will honor the soon-to-be professor emeritus
After Ryland ‘Ry’ Young retires on Aug. 31, his students can keep an ear out for his signature jangling of keys in the hallways — the renowned faculty member will still manage his lab, as professor emeritus.

Young, Ph.D., is the director of the Texas A&M Center for Phage Technology, a University Distinguished Professor, Regents Professor and the Sadie Hatfield Professor of Agriculture in the Texas A&M Department of Biochemistry and Biophysics.
Over his 44-year career at the Texas A&M College of Medicine and College of Agriculture and Life Sciences, Young has made broad advances in the understanding of bacteria-infecting viruses called bacteriophages, or phages. This work was performed in collaboration with many students and colleagues. Overall, Young’s work illuminated the ancient “arms race” between phages and bacteria and shed light on ways to combat antibiotic-resistant bacterial infections.
“I can’t overstate the impact Dr. Young has had on his field, generations of students, this department and the reputation of the university,” said Josh Wand, Ph.D., distinguished professor and head of the Department of Biochemistry and Biophysics. “We want the department to remember him and celebrate those achievements, so we are starting a new endowed lecture series in his honor.”
The Ryland F. Young III Lecture in Biochemistry endowment will support bringing preeminent experts in biochemistry and biophysics to speak at Texas A&M. Donations to the endowment will be accepted for the next five years. The Department of Biochemistry and Biophysics will provide up to $12,500 in matched donations for gifts of $500-$12,500.
Learning about biology from phages

Since starting his faculty career in 1978, Young has focused his work on phages, which have been used to treat or prevent bacterial infections and have also served as an important model organism in biology.
“Phages have about 100 times less genetic material than an average bacterial cell, which has 10 times less genetic information than an average eukaryote,” Young said. “Phages are ‘byte-sized.’ They are easy to manipulate and grow, so it’s easier to ask questions and get definite answers. Most of the time, when you have a hypothesis, it’s wrong — that’s just the nature of science — but it’s nice to find that out quickly.”
After many genetic questions were answered about phages decades ago, interest in using these viruses as model organisms faded in the scientific community. Young watched his field flourish again in recent years when phages offered new ways to control antibiotic-resistant bacteria and opened many questions about gene editing.
New frontiers in gene editing and the phage-bacteria “arms race”
To defend against phages, bacteria have evolved ways to recognize and inactivate phage DNA. When scientists employ the gene-editing tool CRISPR, they are borrowing tricks from the bacterial defense manual against phages.
“The hot news is that phages came up with all kinds of ways of fighting back,” Young said. “Phages came up with many different ways of defeating or taking over the CRISPR systems. Some even turn it around and use CRISPR against other phages.
“If you want to use CRISPR, there are phage proteins to control it, amplify it, slow it down or change its specificity,” he said. “It’s an incredible biological story, an evolutionary story of attack and defense.”
Center for Phage Technology to grow over next five years
In 2010, Young became a founding member and director of the Center for Phage Technology. The center’s mission is to position the Texas A&M University System as a world leader in the application of phages to combat bacterial infections in humans, animals and plants; promote food safety; protect against potential bacteriological weapons; and to prevent or mitigate harmful effects of bacterial contamination, degradation and corrosion.

“The Center for Phage Technology just went through a five-year review, and we’ve been authorized for another five years,” Young said.
The center currently includes seven principal investigators as well as students, staff and scholars. Now, its leadership team plans to expand the center’s membership, Young said.
For example, “bacterial cell biology is phage biology too. Anyone who works on bacteria is part and parcel of the same story,” Young said.
Figuring out how phages make bacteria ‘blow up’
For Young, phages are fascinating aside from their numerous applications in medicine, veterinary science, agriculture and food safety.
After phages infect a bacterial cell and reproduce inside, they can opt to initiate lysis, the breaking of the bacterial cell wall. Lysis kills the bacteria but allows the phages to escape.
“It turns out making bacteria blow up isn’t easy,” Young said. “It’s fascinating on a molecular level because the phages don’t want the cell to blow up immediately. And bacteria have a very robust cell structure. Breaking bacterial cells open and doing it at the right time—how phages do that poses lots of interesting questions in molecular biology and evolutionary biology.”
Discovering relevance in lysis amid combatting antibiotic resistance
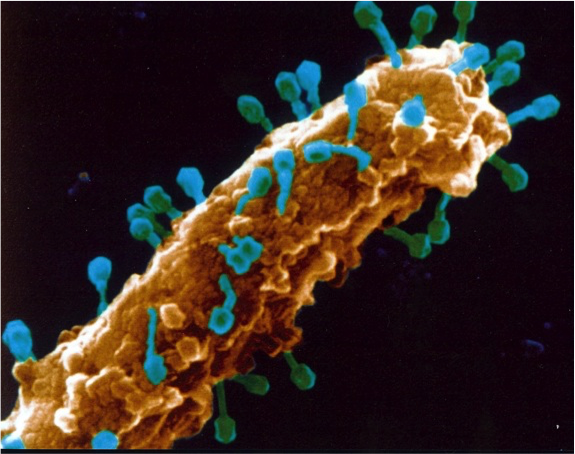
When he was a junior faculty member, Young planned to study the spread of antibiotic resistance. Because he was required to submit grant applications every funding cycle until two grants were funded, he submitted a grant application, almost on a whim, to study how phages initiate lysis.
“My antibiotic-resistance grant didn’t get funded that first time, but the reviewers thought the lysis project was cool,” he said.
The interesting project immediately gripped Young’s attention and eventually turned out to be essential to understanding how phage contend with bacteria.
“This was before phage therapy made a comeback, so no one could show why anyone should care how phage lysis happens,” Young said. “Now, it turns out lysis is the key for phages to be effective antibiotics,” Young said.
Young’s grant to study antibiotic resistance was eventually funded as well. And, he and the Center for Phage Technology became part of a famous success story when they isolated phages to treat a man dying from a multidrug-resistant infection, Tom Patterson, and he recovered.
Hallmarks of a successful career
Young earned his doctorate in molecular biology in 1975 as a National Science Foundation Graduate Fellow at the University of Texas at Dallas. He was a National Institutes of Health, NIH, Postdoctoral Fellow at Harvard Medical School, where he discovered a bacteriophage lambda gene involved in lysis.
In 2003, he was elected as a Fellow of the American Society for Microbiology and a Fellow of the American Academy for the Advancement of Science. He was named the Sadie Hatfield Professor of Agriculture in 2006, a Texas A&M Regents Professor in 2016 and a Texas A&M University Distinguished Professor in 2018.
Young’s teaching and mentorship have garnered the Texas A&M Association of Former Students Distinguished Achievement in Teaching Award, the College of Agriculture and Life Sciences Dean’s Outstanding Achievement Award for Faculty Mentoring, and the Biochemistry Graduate Student Association Special Recognition Award.
Over his career, he has been funded by the NIH for more than 40 uninterrupted years, including an NIH Merit Award. He has authored or coauthored over 160 peer-reviewed articles in top-ranked scientific journals.
Among his many accomplishments over the decades, Young is the proudest of his team’s work to understand lysis.
“We now understand operationally how phage lysis works,” Young said. “We didn’t understand any of that in 1978. Our lab did that, and it will be in textbooks. Along with that, I am proud of the over 40 grad students and postdocs who actually did this work and also went on to successful careers.”
“That’s the real currency of being a teacher, seeing young people learn and progress,” he added. “The lab is kind of a family, but one that’s constantly changing. The nice thing is you have these people who are almost like your children all over the world.”


