Study uncovers aspect of how muscular dystrophies progress
Advance has potential for early diagnosis, treatments of Walker-Warburg syndrome, other muscular dystrophies
A research study has shed new light on how congenital muscular dystrophies such as Walker-Warburg syndrome progress, bringing hope for better understanding, early diagnosis and treatments of these fatal disorders.
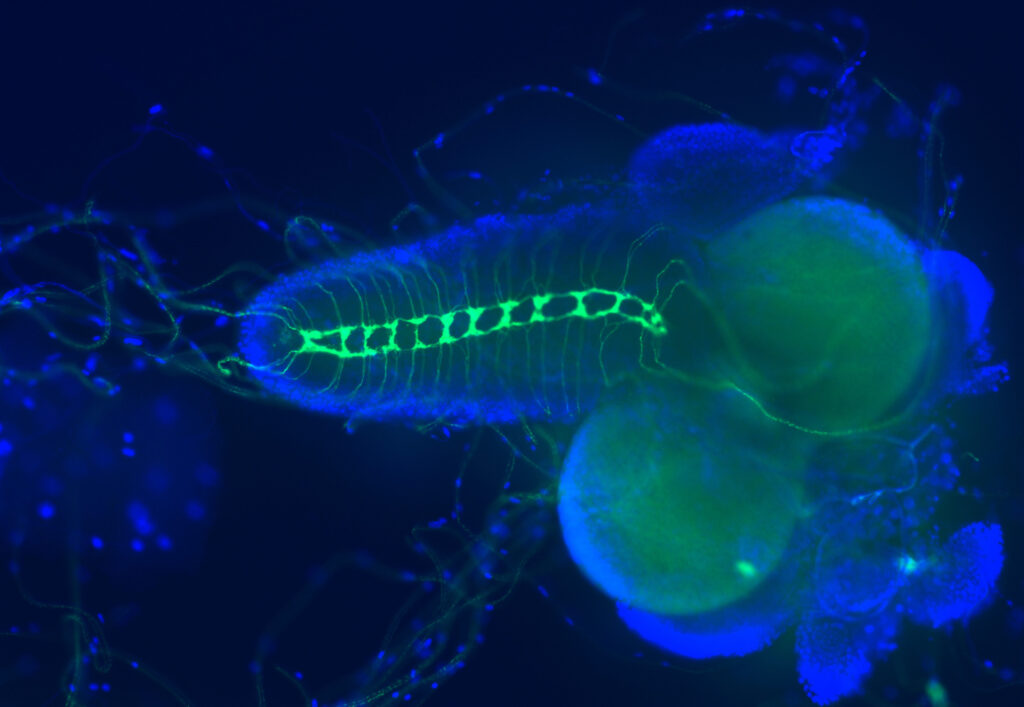
Published in March in the Journal of Biological Chemistry, the research was led by scientists in the lab of Vlad Panin, Ph.D., professor in the Department of Biochemistry and Biophysics in the Texas A&M College of Agriculture and Life Sciences. The study is titled “Protein tyrosine phosphatase 69D is a substrate of protein O-mannosyltransferases 1-2 that is required for the wiring of sensory axons in Drosophila.” The primary author is Pedro Monagas-Valentin, one of Panin’s doctoral students.
The study uncovers new ways of how genetic mutations seen in patients with muscular dystrophies may lead to disease and create neurological problems. Namely, the mutations disrupt a newly discovered gene function and prevent neurons from forming connections properly. The research used fruit flies as a model system and has implications for humans.
Funders of the research included the National Institutes of Health and the Texas A&M AgriLife Institute for Advancing Health Through Agriculture.
Walker-Warburg syndrome and other muscular dystrophies
Walker-Warburg and muscle-eye-brain syndromes are rare, severe muscular dystrophies. Typically diagnosed in very young children, these conditions progress rapidly. They affect skeletal, heart and lung muscles as well as the brain, eyes and other organs. No cure exists for these diseases, and patients usually do not survive into adulthood.
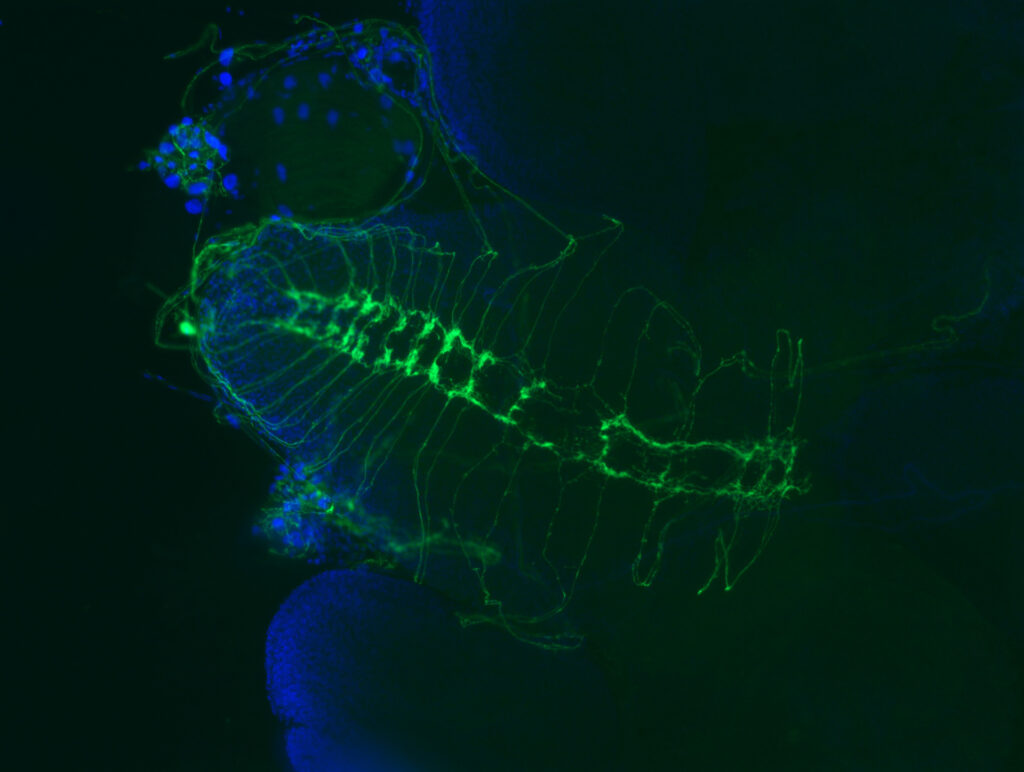
“Certain genes affected in these disorders are known,” Panin said. “But much remains unknown about how these genetic defects affect molecular and cellular processes to cause neurological and other problems.”
This gap in understanding of pathological mechanisms impedes the development of treatments and efficient diagnostics, he said.
A problem tied to sugar biology
Many of the genetic mutations that occur with muscular dystrophies affect something difficult to study, Panin said, and that is the way our bodies build and use complex sugars.
The sugars, called glycans, are made by all living things. In addition to energy storage and regulation, glycans have countless functions that regulate other molecules in animal cells.
“There are four ‘languages’ of life, if you think about it in general,” Panin said. “Two are proteins and nucleic acids like DNA and RNA. And there are two more languages: lipids and glycans. The fourth one is arguably the most complex ‘language,’ and this is what we study as glycobiologists.”
Glycans can be complex and branching. Unlike DNA or proteins, they are not created from a genetic template. The mutations in muscular dystrophy patients disturb a complex chain of events needed to build and attach glycans to the right molecules inside our bodies. To understand that chain of events, scientists must study the structures and locations of glycans, and the technology to do that is still being developed.
What the team did
To track the role of several genetic mutations in muscular dystrophies, the team genetically modified fruit flies, then studied how the mutations affected the flies’ nervous system structure and glycobiology.
“My work involved a lot of crossing different lines of fruit flies to either raise or lower the activity of genes we wanted to learn more about,” Monagas-Valentin said. “Then I did a lot of fly brain dissections under the microscope, of multiple genetic combinations, with a lot of practice and a lot of messing up.”
Monagas-Valentin used fluorescence microscopy and other methods to compare how different mutations in flies affected fly bodies and brains. He also sent samples for analysis using methods specifically designed for glycobiology. For that analysis, Monagas-Valentin and Panin collaborated with researchers at the Complex Carbohydrate Research Center at the University of Georgia in Athens.
“Our collaborators have expertise in glycan sample preparation, data analysis, protocol development — every step is important,” Panin said.
Putting all the data together, the team found that a protein called PTP69D enables the proper wiring of sensory axons in flies. The researchers also revealed that the genes mutated in muscular dystrophy patients are important for PTP69D to function properly. What’s more, PTP69D belongs to a large family of proteins that have very similar structure and function in flies and humans.
“This story opens up new directions to understand neurological problems,” Panin said.
What’s next
Although PTP69D and its protein family members are similar in flies and in humans, there are limitations to what the present study says about human biology, Panin said.
“The fly nervous system is much simpler, and in humans there may be additional protein and glycan interactions in play,” Panin said. “We can see the basic mechanisms, but nuances and additional layers cannot be studied in flies.”
He said much is still unknown about the proteins and glycans involved in neuron development. The team will now study these molecules and interactions more deeply to see how mutations in muscular dystrophy genes affect individual neurons.


